The Chemokine CCL2 Increases Nav1.8 Sodium Channel Activity in Primary Sensory Neurons through a Gβγ-Dependent Mechanism
Por um escritor misterioso
Last updated 19 setembro 2024

Changes in function of voltage-gated sodium channels in nociceptive primary sensory neurons participate in the development of peripheral hyperexcitability that occurs in neuropathic and inflammatory chronic pain conditions. Among them, the tetrodotoxin-resistant (TTX-R) sodium channel Nav1.8, primarily expressed by small- and medium-sized dorsal root ganglion (DRG) neurons, substantially contributes to the upstroke of action potential in these neurons. Compelling evidence also revealed that the chemokine CCL2 plays a critical role in chronic pain facilitation via its binding to CCR2 receptors. In this study, we therefore investigated the effects of CCL2 on the density and kinetic properties of TTX-R Nav1.8 currents in acutely small/medium dissociated lumbar DRG neurons from naive adult rats. Whole-cell patch-clamp recordings demonstrated that CCL2 concentration-dependently increased TTX-resistant Nav1.8 current densities in both small- and medium-diameter sensory neurons. Incubation with CCL2 also shifted the activation and steady-state inactivation curves of Nav1.8 in a hyperpolarizing direction in small sensory neurons. No change in the activation and inactivation kinetics was, however, observed in medium-sized nociceptive neurons. Our electrophysiological recordings also demonstrated that the selective CCR2 antagonist INCB3344 [ N -[2-[[(3 S ,4 S )-1- E 4-(1,3-benzodioxol-5-yl)-4-hydroxycyclohexyl]-4-ethoxy-3-pyrrolidinyl]amino]-2-oxoethyl]-3-(trifluoromethyl)benzamide] blocks the potentiation of Nav1.8 currents by CCL2 in a concentration-dependent manner. Furthermore, the enhancement in Nav1.8 currents was prevented by pretreatment with pertussis toxin (PTX) or gallein (a Gβγ inhibitor), indicating the involvement of Gβγ released from PTX-sensitive Gi/o-proteins in the cross talk between CCR2 and Nav1.8. Together, our data clearly demonstrate that CCL2 may excite primary sensory neurons by acting on the biophysical properties of Nav1.8 currents via a CCR2/Gβγ-dependent mechanism.

Chemokine CCL7 mediates trigeminal neuropathic pain via CCR2/CCR3-ERK pathway in the trigeminal ganglion of mice - Lin-Peng Zhu, Meng-Lin Xu, Bao-Tong Yuan, Ling-Jie Ma, Yong-Jing Gao, 2023

PDF] Phosphorylation of Sodium Channel Nav1.8 by p38 Mitogen-Activated Protein Kinase Increases Current Density in Dorsal Root Ganglion Neurons

TRPV 1 receptor inhibition decreases CCL 2-induced hyperalgesia
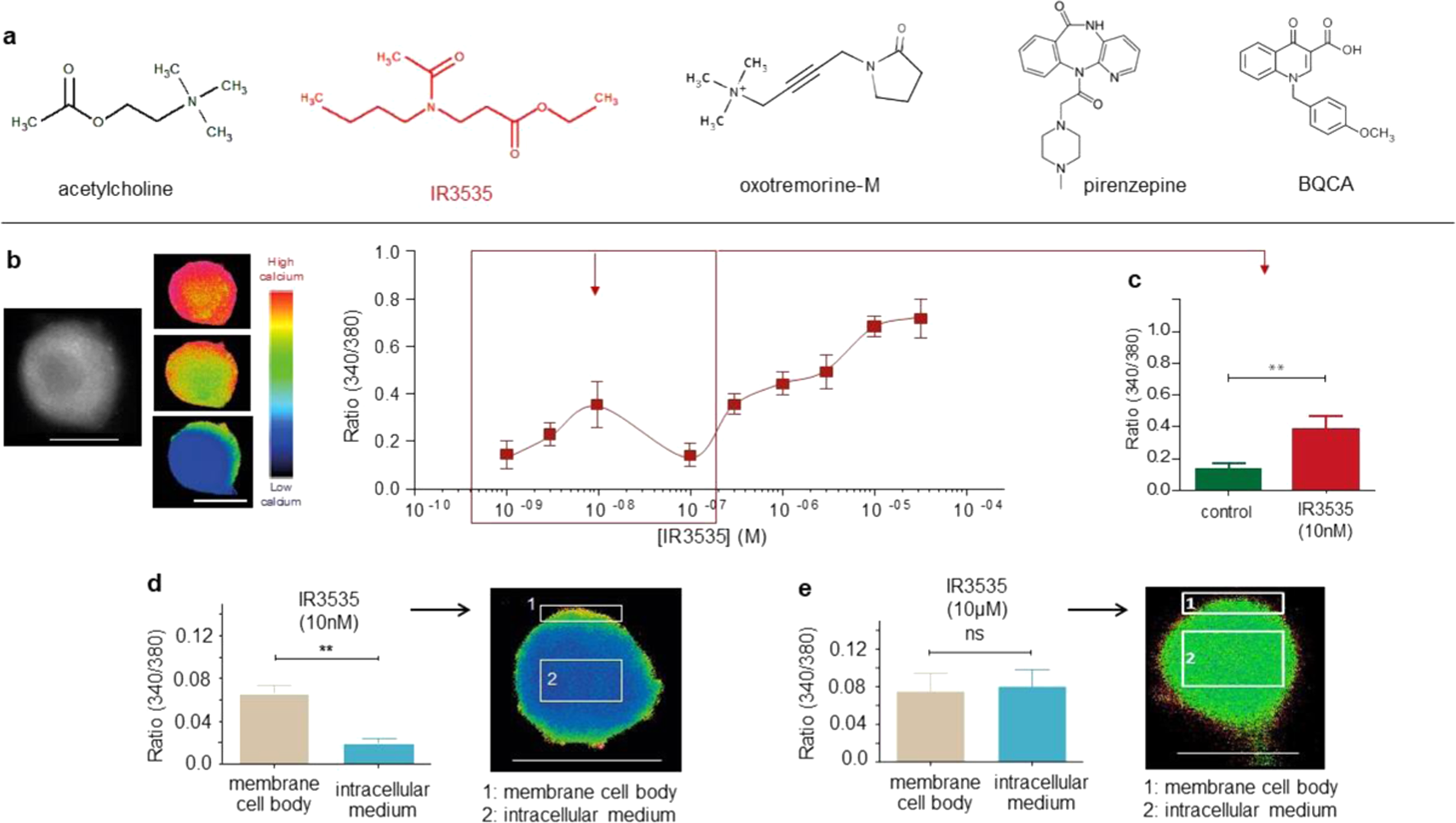
Orthosteric muscarinic receptor activation by the insect repellent IR3535 opens new prospects in insecticide-based vector control
PDF) Functional up-regulation of Nav1.8 sodium channel in Aβ afferent fibers subjected to chronic peripheral inflammation
The analgesic activities of Stauntonia brachyanthera and YM11 through regulating inflammatory mediators and directly controlling the sodium channel prompt

Therapeutic Opportunities and Challenges in Targeting the Orphan G Protein-Coupled Receptor GPR35

Implication of the chemokine CCL2 in trigeminal nociception and traumatic neuropathic orofacial pain - Dauvergne - 2014 - European Journal of Pain - Wiley Online Library

Functional inhibition of chemokine receptor CCR2 by dicer-substrate-siRNA prevents pain development - Valérie Bégin-Lavallée, Élora Midavaine, Marc-André Dansereau, Pascal Tétreault, Jean-Michel Longpré, Ashley M Jacobi, Scott D Rose, Mark A Behlke

Conditional Ablation of Astroglial CCL2 Suppresses CNS Accumulation of M1 Macrophages and Preserves Axons in Mice with MOG Peptide EAE
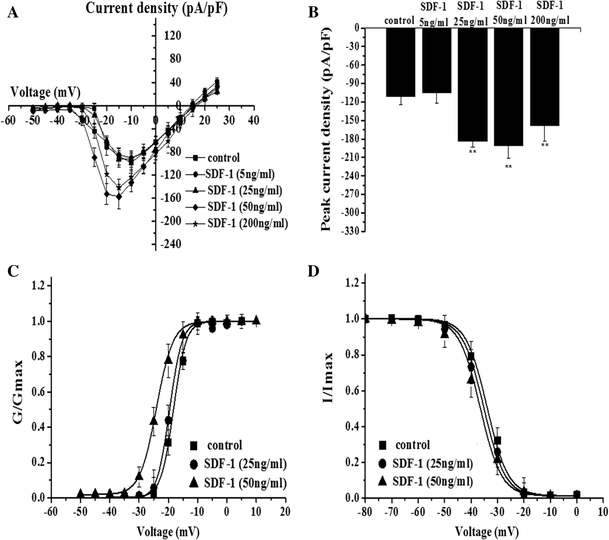
Stromal Cell-Derived Factor 1 Increases Tetrodotoxin-Resistant Sodium Currents Nav1.8 and Nav1.9 in Rat Dorsal Root Ganglion Neurons via Different Mechanisms

Neonatal Injury Modulates Incisional Pain Sensitivity in Adulthood: An Animal Study - ScienceDirect
IJMS, Free Full-Text

The Chemokine CCL2 Increases Nav1.8 Sodium Channel Activity in Primary Sensory Neurons through a Gβγ-Dependent Mechanism

The Chemokine CCL2 Increases Nav1.8 Sodium Channel Activity in Primary Sensory Neurons through a Gβγ-Dependent Mechanism
Recomendado para você
-
Lavoisier - RT-PCR: O exame mais preciso para COVID-19 no19 setembro 2024
-
 Sintomas de pneumonia: quais são, diagnóstico e tratamento19 setembro 2024
Sintomas de pneumonia: quais são, diagnóstico e tratamento19 setembro 2024 -
 Diagnósticos da América – Wikipédia, a enciclopédia livre19 setembro 2024
Diagnósticos da América – Wikipédia, a enciclopédia livre19 setembro 2024 -
 Os 10 principais modelos de scorecard de avaliação de funcionários19 setembro 2024
Os 10 principais modelos de scorecard de avaliação de funcionários19 setembro 2024 -
 Landmark Events — History Highlight — Antoine-Laurent Lavoisier Born, 174319 setembro 2024
Landmark Events — History Highlight — Antoine-Laurent Lavoisier Born, 174319 setembro 2024 -
 Nav Projects Photos, videos, logos, illustrations and branding on Behance19 setembro 2024
Nav Projects Photos, videos, logos, illustrations and branding on Behance19 setembro 2024 -
Genius, Hawking, and expertise - Michael Lynch, 201419 setembro 2024
-
 Outubro Rosa Dasa: Download gratuito da revista Capricho19 setembro 2024
Outubro Rosa Dasa: Download gratuito da revista Capricho19 setembro 2024 -
 About Mercury19 setembro 2024
About Mercury19 setembro 2024 -
 The Evolution of Knowledge19 setembro 2024
The Evolution of Knowledge19 setembro 2024
você pode gostar
-
 Porta Cartas Uno - Trinity 3D - Transforme suas ideias em realidade!19 setembro 2024
Porta Cartas Uno - Trinity 3D - Transforme suas ideias em realidade!19 setembro 2024 -
![MCQ] If α and β are the zeros of a polynomial f(x) = px2 – 2x + 3p](https://d1avenlh0i1xmr.cloudfront.net/5c6616c9-a254-4cb6-b0aa-2b54f21e9cb3/slide6.jpg) MCQ] If α and β are the zeros of a polynomial f(x) = px2 – 2x + 3p19 setembro 2024
MCQ] If α and β are the zeros of a polynomial f(x) = px2 – 2x + 3p19 setembro 2024 -
 Assistir Kami-tachi ni Hirowareta Otoko Dublado - Todos os Episódios19 setembro 2024
Assistir Kami-tachi ni Hirowareta Otoko Dublado - Todos os Episódios19 setembro 2024 -
 illaoi from league of legends 3D Print Model in Woman 3DExport19 setembro 2024
illaoi from league of legends 3D Print Model in Woman 3DExport19 setembro 2024 -
 Jogo Sword Art Online - Lost Song Playstation 4 Ps4 Novo Top em Promoção na Americanas19 setembro 2024
Jogo Sword Art Online - Lost Song Playstation 4 Ps4 Novo Top em Promoção na Americanas19 setembro 2024 -
Grognougnou (@Grognougnou) / X19 setembro 2024
-
 Showroom - Araranguá19 setembro 2024
Showroom - Araranguá19 setembro 2024 -
 Assassin's Creed: Revelations - Download19 setembro 2024
Assassin's Creed: Revelations - Download19 setembro 2024 -
 PDF) Fernando Pessoa e a Tradução19 setembro 2024
PDF) Fernando Pessoa e a Tradução19 setembro 2024 -
 How Does 'The Legend of Zelda: Tears of the Kingdom' Run On Switch?19 setembro 2024
How Does 'The Legend of Zelda: Tears of the Kingdom' Run On Switch?19 setembro 2024